JD Investment Insights:“Micro-organ” in Petri dish is supporting another potential track in biomedical industry?
2023-06-29
Abstract: There is a wide range of application scenarios for organoid, and the industry has a large space for development.
Pancreatic cancer is known as the “King of cancers”. The founder of Apple, Steve Jobs, Nobel Prize winner Steinman, tenor Pavarotti, and Hong Kong artist Dianxia Shen have all lost their lives to this disease.
Pancreatic cancer starts insidiously and progresses rapidly, often reaching advanced stages once diagnosed, and some patients even have only a few months to survive after diagnosis. The use of organoid may turn the fate of pancreatic cancer patients around.
Organoid refers to a tissue analog with a certain spatial structure formed by using adult stem cells or pluripotent stem cells cultured in vitro in three dimensions (3D). Although they are not truly human organs, they are called "micro-organs" because they can mimic real organs in terms of structure and function.
The oncology information platform “Guideline Interpretation” has shared a pancreatic cancer case: a 75-year-old male patient with a diagnosis of mucinous adenocarcinoma of the pancreas. As the cancer had metastasized, the patient was unable to undergo radical treatment through surgery, and the only way to prolong life and improve quality of life was to look forward to drug treatment.
In order to find the appropriate drug as soon as possible, clinicians extracted the patient's ascites (peritoneal fluid) for tumor organoid culture and drug sensitivity testing.
The results showed that the combination of trametinib + hydroxychloroquine had the highest inhibition rate of cancer cells in this patient's tumor organoids among all alternative drug regimens. Therefore, the clinician changed the treatment regimen for this patient to oral trametinib + hydroxychloroquine.
After the first course of treatment, the patient's ultrasound examination showed that the ascites was clearly under control and the margins of the pancreatic tumor were wrinkled; 6 weeks after taking the medication, the patient's appetite and mental state had also greatly recovered, he gained 3kg and climbed a mountain on his own. This is a miracle for the patient who was once in pain, extremely thin and bedridden.
Precision medicine, including drug sensitivity testing, is one of the more mature applications of organoids. In addition, organoids have applications for new drug development, gene editing, organ transplantation, as well as infection biology, toxicology research, and even public health and safety and military fields.
As a cutting-edge biotechnology, organoid has been recognized by the international academic community. 2013, organoid was selected by Science as one of the top ten technological advances; In 2017, organoid was named technology of the year in life science by Nature Methods; In 2021, the organoid-based malignant tumor disease model was listed as one of the first batches of key special tasks to be launched in China's 14th Five-Year Plan.
In the industry, the entire organoid industry is still in its infancy. The data shows that the global organoid market size was around $700 million in 2019 and is expected to reach $3.4 billion by 2027, growing at a CAGR of around 22%.
JD Capital learned in the survey that in China's organoid industry, relevant companies are concentrated to be established in 2018-2021, and most of the current financing stages are in an angel round to Pre-A rounds. Even for the leading companies, the annual revenue volume is still at the 10 million level.
In foreign countries, organoid has become an area where big pharmaceutical companies are competing for positions.
Since 2015, Johnson & Johnson, Merck, Pfizer, and Sanofi have introduced organoid technology in new drug development; AbbVie, Merck, and Novartis have jointly established the Innovation and Quality Consortium, a nonprofit organization that aims to promote the standardized application of organoid chips.
As recently as May 2023, Roche also announced the establishment of the Institute of Human Biology (IHB) to work on human model systems such as organoids.
Currently, the faster commercialization applications of organoid are clinical-level personalized drug sensitivity testing on the 2C (To customer) side and new drug development on the 2B (To business) side. However, JD Capital judges that organoid, as a newly emerged segment from the biopharmaceutical industry, has a wide range of application scenarios and a large space for industry development.
Of course, for any emerging industry, opportunities always coexist with risks.
1.A better drug-testing stand-in than a monkey
Safety evaluation is an important part of drug development, and preclinical safety evaluation is necessary to prevent toxic drugs from entering clinical trials. For a long time, animal testing has been an important tool for preclinical safety evaluation.
This recognition generally began in 1938 when President Roosevelt signed the Federal Food, Drug and Cosmetic Act, which required evidence of safety before all drugs could be marketed. New drugs must be tested on animals before they can be tested clinically in humans.
With the development of modern medicine, testing on non-human primates (monkeys) is the closest to human testing.
Animal testing, however, faces controversy, such as the fact that it is against humanitarianism for animals to be subjected to high levels of pain during testing; animal testing also has potential safety issues, such as the possibility of animals entering the market after testing is completed, causing the spread of viruses and bacteria and outbreaks.
In 1959, some zoologists and microbiologists proposed the 3R principle to solve the above problems of animal testing, i.e., replacement, optimization, and reduction.
Even earlier, more than 100 years ago, attempts were made to culture animal tissues in vitro. In the 1980s, 3D organoid models of primary tissues cultured in vitro became available, laying the foundation for organoid research. In 2009, the first intestinal organoid that could be cultured and went down to the next generation for long periods of time was constructed by the team of Dutch scientist Hans Clevers.
Currently, a variety of organoids have been successfully constructed, including small intestine, stomach, colon, lung, bladder, brain, liver, pancreas, kidney, ovary, esophagus, and heart, containing both normal organ tissue organoids and corresponding tumor tissue organoids.
JD Capital has learned that there are several advantages of using organoids for drug trials compared to animals: shorter culture period, cheaper, and the test data is closer to clinical data.
On the one hand, animal testing has specific requirements for the age of animals (monkeys usually require three years of growth before they can be used for testing), long breeding cycles, and low yield rates. On the other hand, almost all commercial airlines have refused to transport such animals in recent years.
This had led the domestic and international markets to a predicament where it was difficult to find test monkeys, and the unit price of test monkeys then soared all the way up. In the case of the most used crab-eating monkey, for example, its unit price rose from less than ¥7,000 in 2017 to more than ¥180,000 in 2022, an increase of more than 20 times. Some companies in the industry need to wait six months if they want monkeys.
In new drug R&D, according to industry sources, under the condition that no monkeys are used and, and compound generation is not included, the price quoted by domestic CROs (pharmaceutical R&D outsourcing companies) from target discovery to clinical declaration is around ¥50 million; if monkeys are used for testing, the price will be more expensive; if organs of the class are used to complete the process, the price can be saved to around ¥25 million.
As for the trial data, the CEO of an organoid company revealed that the company had tested hepatotoxicity ratings on 122 approved marketed drugs in liver models. The results showed that the positive prediction rate of liver model organoids could reach more than 85% compared with clinical data, which is 30 percentage points higher than that of non-human primate models.
In terms of drug sensitivity testing, according to JD Capital's research, the current price for a single person to conduct organoid drug sensitivity testing is around ¥20,000, and the common service cycle in the industry is 14 days, including the whole process: sampling, transportation, culture, verification, testing, analysis, and reporting.
Organoid culture is the core part of the organoid technology service process. The classical route is to culture the isolated stem cells on 3D scaffolds (e.g., Matrigel), spread in petri dish or culture orifice, and add various growth factors, including FGF (fibroblast growth factor) and EGF (epidermal growth factor) to promote organoid formation.
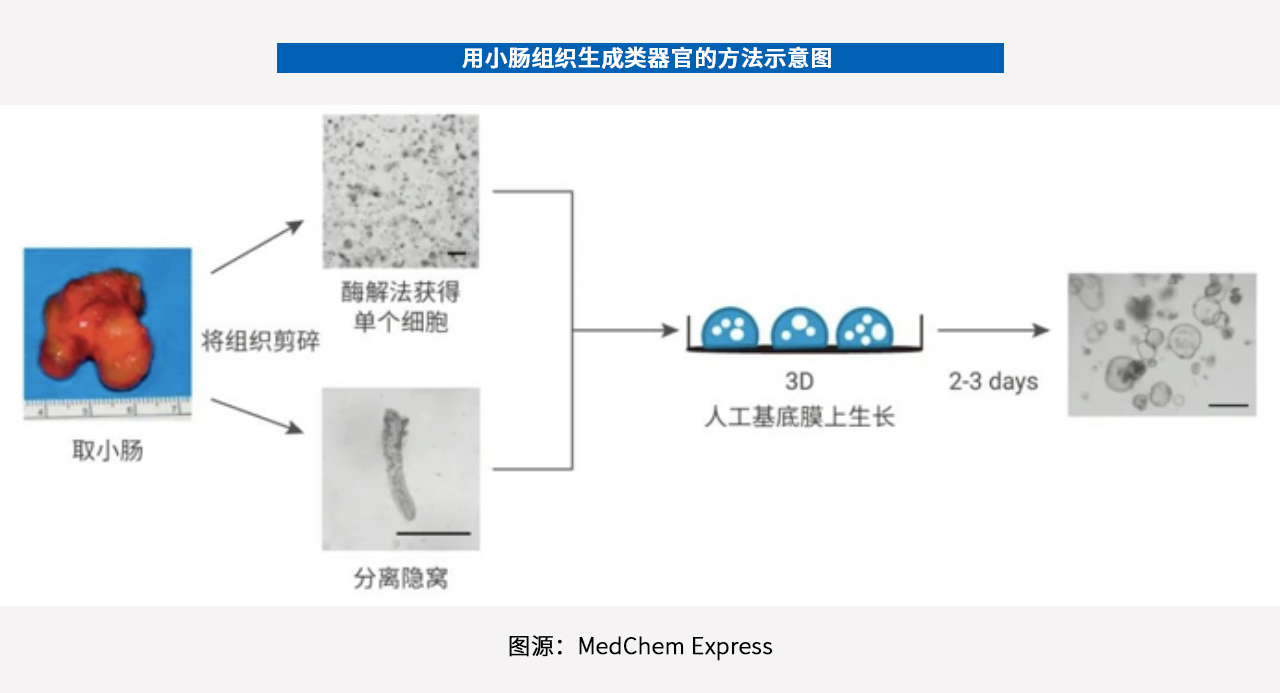
Organoid features also include the ability to pass on (often referring to the re-separation of proliferating cell populations, transferring cells from one culture vessel to another), freeze storage, resuscitation, gene editing, and stable genetic information.
Organoids that can be passaged, mostly undifferentiated, can be passaged 20-30 times based on different tissues and culture protocols. After 2-3 passages, the organoids can be stored frozen in liquid nitrogen. For best results, the organoid is generally selected for freezing after maturation (7-10 passages).
2.Dilemmas and challenges in the tide of opportunities
Perhaps realizing the increasing substitutability of animal testing and seeing the potential of related technologies, the FDA (Food and Drug Administration) Modernization Act 2.0 came out of nowhere.
The FDA Modernization Act 2.0 is an amendment to the Federal Food, Drug, and Cosmetic Act and the Public Health Service Act that authorizes the use of certain alternatives to animal testing (such as cell-based assays, microphysiological systems, bioprinting, or computer models) to demonstrate the safety and efficacy of drugs.
In September 2022, the U.S. Senate passed the FDA Modernization Act 2.0 by unanimous consent, and in December of that year, the bill was signed into law by President Biden as part of the Consolidated Appropriations Act.
And the version of the bill passed by the U.S. House of Representatives in June 2022 - the H.R. 7667 - Food and Drug Amendments of 2022. For the first time includes organ-on-a-chip and microphysiological systems as independent systems for evaluating nonclinical trials of drugs. And is seen as an equally important research tool as cell-based assays, computer modeling, and animal models.
Organ-on-a-chip, which is based on the microfluidic chip on which human organ physiological microsystems are constructed, can be understood as: a short version of human tissues and organs constructed outside the human body by a U disk-sized chip. Organ chips are generally composed of three parts: cells obtained from humans or animals; skeletal materials used to form in vitro models; and bioreactors used to simulate the growth environment of organs in vivo.
Scientists use cells from animals or humans to be cultured and then assembled into organ chips. It can not only test the effectiveness and safety of drugs, but also be used to study the dysfunction and pathogenesis of real organs.
Taking the liver chip as an example, all food and drugs ingested into the body have to be broken down by the liver before excretion, and the damage or effectiveness caused by the drug will be reflected in the liver. Therefore, when an experimental drug passes through the human liver chip to damage cells, the chip will issue a toxicity warning.

Organ-on-a-chip has some similarities to organoid, but it is `another technology route developed independently.
The organoid is biased toward biology, with the advantage of high simulation and highly similar histological features and functions to human organs, but has limitations in controllability and reproducibility.
Organ chips are biased toward biomedical engineering and have advantages in controllability and standardization of modeling, and can achieve the construction of more complex models through co-culture technology; however, organ chip models constructed from a single type of cells are not biomimetic enough in terms of biology.
The Organoids-on-Chips, which is considered to integrate the advantages of both of two technological routes.
In August 2022, the FDA approved the world's first new drug to enter clinical trials based entirely on preclinical data obtained from Organoids-on-Chips studies. The new drug trial is a collaboration between Sanofi and organoid chip company Hesperos for the treatment of two rare autoimmune demyelinating neurological disorders. Previously, the industry has been unable to conduct studies for these diseases due to a lack of ideal animal models.
After the FDA Modernization Act 2.0 took legal effect, the news was transmitted to the capital markets and industry with a very rapid and unstoppable momentum.
In January 2023, Emulate, a U.S. organ chip and ancillary device development company, announced the terms of an IPO with an estimated market capitalization of approximately $125 million. Previously, the company has completed multiple rounds of financing totaling nearly $225 million, making it the most financed company in the organ-on-a-chip segment.
A number of new financing rounds have also been completed by organoid-related companies in China so far in the second half of 2022.
The rapid emergence of organoid-related technologies as a new opportunity is also due to the driving force from China. For example, in June 2022, the Expert Consensus on Organoid Drug Sensitivity Testing to Guide Clinical Applications in Tumor Precision Therapy, which was co-authored by several clinical experts, was released, further promoting the application of organoid in tumor precision therapy.
JD Capital observed that in an emerging industry, Chinese organoid-related companies are also facing dilemmas and challenges, including:
1.Upstream materials are restricted. At present, the materials of organoid products (such as matrix glue, growth factors needed to maintain the ecology and differentiation of organoid, cell culture plates, etc.) are mostly in the state of import monopoly. If companies can achieve the import substitution of materials, the cost is expected to drop significantly.
2.Market education and promotion need to be continued. Although more and more doctors and R&D personnel are accepting and recognizing organoid-related technologies, there is no necessary connection with whether they can be transformed into commercial orders. Currently, there is no unified standard in the product side of the industry, so it is difficult for companies to promote the market.
3.Relative lack of talents. According to the rough estimation of JD Capital, there are about 20 companies carrying out organoid business. According to 3~5 core R&D personnel per company, there are less than 100 core R&D personnel in the whole industry. Moreover, the industry is in the early stage of market education and development, and the mobility of core personnel is relatively large.
4. Since the regulation of the organoid industry is not yet sound, the business model of enterprises is mainly based on LDT (Laboratory Developed Test). Under the LDT model, organoid products cannot be licensed and are difficult to enter hospitals and cannot be reimbursed by medical insurance.
3.How to grasp the opportunity of the emerging segmentation track?
There are four main business models for organoid companies - research collaboration, organ chip sales, in vitro diagnostics, and new drug development. The latter two are currently mainstream in China, but are not conducted in the same mode.
In the in vitro diagnostic business, organoid companies are divided into LDT and IVD (In Vitro Diagnostic products). The difference is that LDT is more service oriented, and IVD is more product oriented; LDT services do not require regulatory approval, while IVD products are subject to regulatory approval; LDT is for hospitals, pharmaceutical companies and third-party testing organizations that do not have testing capabilities, while IVD is for medical institutions that have testing capabilities.
JD Capital is aware that the organoid in vitro diagnostic business has not yet reached the level of IVD products and can only provide services in the form of LDT at the present. In the future, with the sound regulation of the industry, the LDT services of enterprises will be gradually standardized and the data indicators of clinical effectiveness will be accumulated for product registration and approval and certification, which can be turned into IVD products.
As an in vitro drug testing tool, organoids can complement NGS (Next Generation Sequencing, also known as high-throughput sequencing or second-generation sequencing), which determines whether a drug is suitable for a patient at both the cellular and genetic levels.
Using the organoid, researchers can screen for multiple drugs or different concentrations of drugs on the petri dish, enabling multiple experiments to be conducted simultaneously.
In the new drug R&D business, organoid companies are more like preclinical CROs (for more details on CXO, read CXO: Can the "shovel seller" in the pharmaceutical field build a world-class company?) For this type of collaboration, pharmaceutical companies are mainly concerned about the stability and heterogeneity of the organoid, the richness of the organoid library, and the availability of the required disease models.
In addition to NGS, organoid and AI drug development are also synergistic and will greatly accelerate the progress of new drug development.
Currently, the U.S. is ahead of China in terms of underlying organoid technology. In terms of organoid industry development, China is faster than the US. The US organoid companies are mostly serving the new drug development of big pharmaceutical companies, while Chinese companies have a richer business model.
Through interviews, JD Capital learned that the development of organoid LDT business in the U.S. is relatively slow for three main reasons: First, the U.S. has strict protection of patient samples and information. Secondly, the qualification requirements for such testing service providers in the U.S. are strict. Third, such fee-based services are subject to whether the U.S. health insurance companies include them in the reimbursement, and if they do not, the general public can hardly afford the price.
In the case of Chinese organoid companies, future competition will revolve around the following dimensions:
1. Maturity of organoid-related underlying technologies
Organoid-related underlying technology involves quality control in the process of sample sampling, preservation and transportation, organoid culture, and drug sensitivity testing.
According to JD Capital's research, the indicators for evaluating the quality of organoid include: cell morphology, quantity, diameter, days of culture, etc. The criteria for evaluating the success of organoid culture are: organoid and its source tissue are highly consistent in pathology and genetics, and can proliferate and pass on stably in vitro. By corporate standards, the success rate of organoid culture in the industry is generally above 80%.
From an investment perspective, companies with leading technology levels are favored.
2. Abundance and compliance of organoid libraries
For companies, the threshold of cell sample acquisition and library construction is high. Especially in the field of new drug research and development, clients are more concerned about the richness of organoid sample libraries. JD Capital learns that the sample libraries of organoid companies are generally small at present, with the number of samples ranging from a few hundred to thousands.
Like cell repositories, organoid sample repositories are differentiated into national, hospital, and corporate levels depending on their qualifications. Most companies build their sample banks based on their own processes and quality standards, and have problems with poor sample quality (e.g., insufficient cell activity) due to differences in sampling techniques, transportation methods, and preservation environments.
According to JD Capital's research, pharmaceutical companies nowadays cooperate with organoid companies more for the purpose of validating internal R&D processes, and the compliance requirements for organoid sample libraries are not strict. However, in order to be used in clinical applications, organoid sample libraries need to be reviewed by the China Institute of Food and Drug Control (CIFDC).
We expect that industry quality standards on organoid sample banks will emerge in the future. In the long term, high-quality sample banks validated by compliance will become an important factor in determining the competitive landscape of the industry.
3. Commercial application scenario coverage
Currently, the main application scenarios of organoid are in vitro diagnosis (especially tumor clinical drug sensitivity testing) and new drug development.
In the judgment of JD Capital, there are two types of companies with greater advantages: one is the company that carries out NGS or NIPT (non-invasive prenatal testing) early tumor screening under the original LDT model, and the other is the company that carries out clinical CRO services. They generally have direct access to clinical endpoints resources (i.e. tertiary hospitals and mainstream oncologists), and it is logical to bridge the organoid business.
In the future, with the expansion of organoid applications in the field like infection biology, organ regeneration and transplantation, the more commercial application scenarios covered, the more competitive the company will be in the future.
*Exchange more projects or ideas, welcome to contact: zhangyf@jdcapital.com
